Introduction: GLP-1 RA Litigation
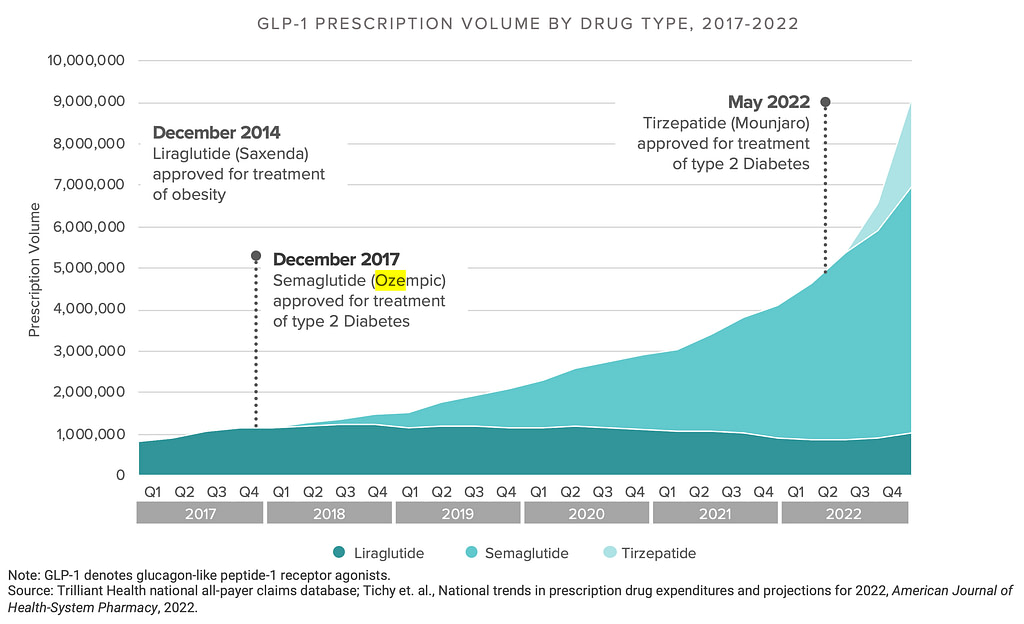
Ozempic and other Semaglutide based drugs have seen blockbuster growth due to its effectiveness in weight loss. MDL No. 3094 is litigation against manufacturers of GLP-1 receptor agonists (GLP-1 RA), such as Ozempic®, Wegovy®, Saxenda®, Trulicity®, Rybelsus®, and Mounjaro®, for causing severe gastrointestinal side effects misrepresented as safe by the manufacturers. The plaintiffs allege that these medications led to gastroparesis (stomach paralysis), ileus (lack of movement in the intestines), and intestinal pseudo-obstruction, despite the manufacturers’ awareness of these risks. The suits claim the manufacturers failed to provide adequate warnings to both doctors and patients about the severity of these risks, focusing instead on extensive direct-to-consumer marketing campaigns that highlighted benefits while downplaying or omitting the severe gastrointestinal side effects.
In this report, we will estimate the number of patients that may have experienced the adverse events described above and may have a potential claim against the manufacturers of GLP-1 receptor agonists.
FDA FAERS Adverse Event Reporting System
The FDA Adverse Event Reporting System (FAERS) is an extensive database crafted for capturing reports of adverse events, medication errors, and quality complaints related to drugs and therapeutic biologics. It’s a cornerstone in the FDA’s safety monitoring activities after a drug has been marketed, ensuring compliance with international safety norms. This platform aggregates inputs from healthcare professionals, consumers, and manufacturers. Reports can be submitted directly to the FDA by healthcare providers such as doctors and pharmacists, as well as by patients and their families. Additionally, manufacturers are legally obligated to report any adverse events they learn about from healthcare professionals or consumers. Within FAERS, adverse events are categorized into serious and non-serious reports.
This database enables us to estimate the potential number of patients who may have experienced adverse effects from the use of GLP-1 receptor agonists like semaglutide, liraglutide, tirzepatide, and dulaglutide between 2014 and 2024 by analyzing the serious adverse events reported during this period. If you want to use a 2019-2023 time frame, reduce the estimates below by 35%.
Analysis: MDL No. 3094 delves into conditions such as gastroparesis, ileus, and pseudo-intestinal obstruction, which are challenging to distinguish due to their similar symptoms, including vomiting, nausea, and constipation. We have pinpointed the key symptoms for each condition through comprehensive reviews of university and hospital websites, alongside examining the adverse events potentially linked to them:
- Gastroparesis is characterized by symptoms such as nausea, vomiting, abdominal pain, constipation, abdominal discomfort, impaired gastric emptying, eructation, abdominal distension, gastrointestinal disorders, GERD, and retching.
- Ileus presents with nausea, vomiting, abdominal pain, constipation, abdominal distension, ileus itself, gastrointestinal pain, paralytic ileus, abnormal gastrointestinal sounds, and intestinal pseudo-obstruction.
- Intestinal obstruction manifests through diarrhea, nausea, vomiting, abdominal pain, constipation, abdominal discomfort, abdominal distension, small and large intestinal obstruction, gastrointestinal pain, and abnormal gastrointestinal sounds.
Serious Outcome by Category
We can then summarize these serious adverse events by categories. Please note that a report may contain one or more outcomes.
| Serious Outcome | Number of Cases |
|---|---|
| Died | 131 |
| Disabled | 214 |
| Hospitalized | 2,375 |
| Life Threatening | 159 |
| Required Intervention | 118 |
| Other Outcomes | 3,287 |
Estimating Potential Injuries
According to the FDA, the time required to complete AE reporting forms is estimated to be 40 minutes which may limit voluntary reporting, it is estimated that only 1-10% of significant AEs are reported by physicians. In addition to that, not all patients report their AEs to their physician.
As of February 25, 2023, there have been 5,316 reports of serious adverse events submitted to the FDA. Among these reports, 131 individuals have died, 214 have become disabled, and 2,375 have required hospitalization. It’s important to note that a single report may place a patient in one or more of these categories. For instance, a patient may be hospitalized due to an adverse event and subsequently pass away.
Analysis of Adverse Events by Death, Disability or Hospitalization
For the purposes of our analysis and to adhere to a more conservative approach, we focus only on the outcomes of death, disability, or hospitalization. This approach simplifies the categorization of adverse events and is in line with the more stringent criteria often employed by attorneys in these cases. Moreover, based on the FDA’s estimates, we adopt conservative and aggressive projections that only 10% and 5%, respectively, of actual adverse events are reported.
| Serious Outcome | Number of Cases | 10% Reporting Rate | 5% Reporting Rate |
|---|---|---|---|
| Died | 131 | 1,310 | 2,620 |
| Disabled | 214 | 2,140 | 4,280 |
| Hospitalized | 2,375 | 23,750 | 47,500 |
| Totals | 2,720 | 27,200 | 54,400 |
Analysis of Adverse Events by Total Serious Reports Received
If we want to apply a looser criteria of all unique reports of serious outcomes received along with the assumption of a 10% and 5% reporting rates of actual adverse events being reported, we come up with the following estimates:
| Number of Serious Outcomes | 10% Reporting Rate | 5% Reporting Rate |
|---|---|---|
| 5,316 | 53,160 | 106,320 |
Summary
We estimate that 27,200 to 54,400 serious adverse events consisting of death, disability or hospitalization occurred where GLP-1 RA inhibitors were considered the suspect product. If your criteria includes all serious outcomes as defined by the FDA, we estimate the total number of potential claimants in the 53,160 to 106,320 range.
Growing Your Docket
If you are interested in growing your docket, we can generate qualified intakes for your firm. Here’s how we reduce fraud and maximize your marketing investment:
- Identify Verification
- Medical Verification (optional)
- US-Based Call Center
- Guaranteed 100% Contact Rate
- TCPA/CAN-SPAM Compliant